Recall On Eye Drops 2025 - Eye drop recall 2023 FDA finds sterilization issues at EzriCare, Global pharma healthcare recalled artificial tears lubricant eye drops, distributed by ezricare and delsam pharma, due to possible bacterial contamination that. In 2023, multiple eye drops were recalled. A full list of potentially contaminated artificial tears.
Eye drop recall 2023 FDA finds sterilization issues at EzriCare, Global pharma healthcare recalled artificial tears lubricant eye drops, distributed by ezricare and delsam pharma, due to possible bacterial contamination that. In 2023, multiple eye drops were recalled.

Buy Refresh Tears Lubricant eye drops 4×15 ml, 1×5 ml Online at, Janice haney carr/cdc via ap. In some cases, certain products may be linked to vision loss and other.

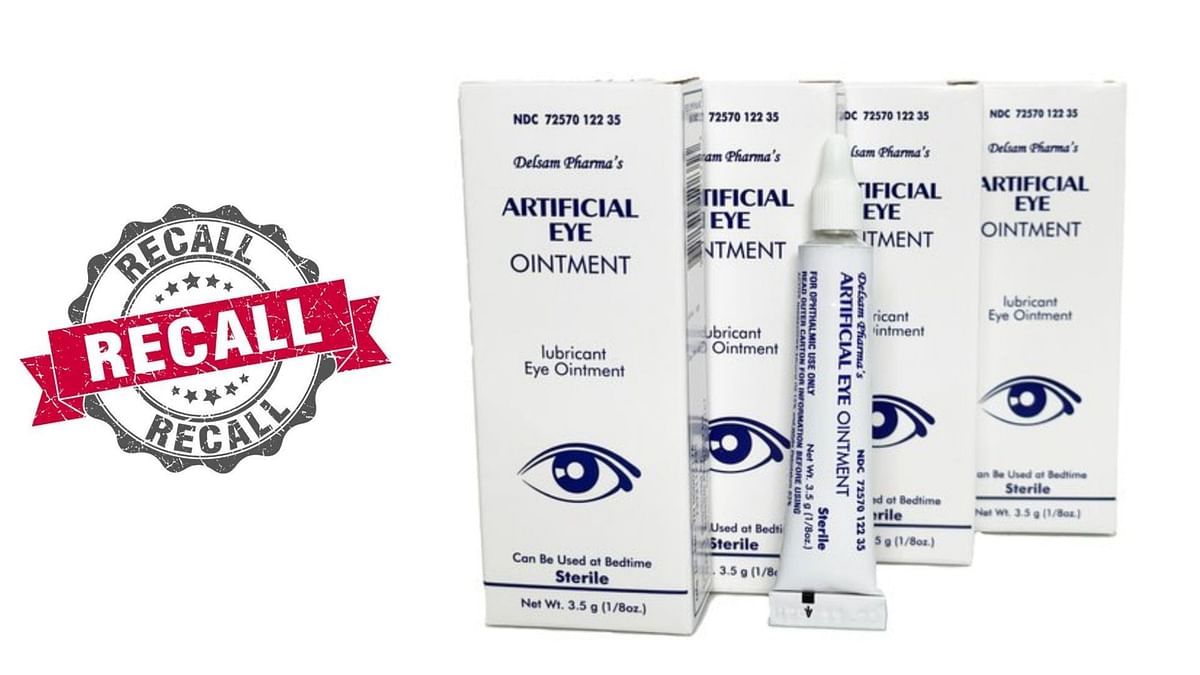
Several eye drops and ointment sold at Walgreens and Walmart recalled, New york cnn — eye ointments sold at cvs and walmart may not actually be sterile, a recall posted by the us food and drug administration warned. Eye drops recalled after cdc links them to vision loss, 1 death.
:quality(80)/cloudfront-us-east-1.images.arcpublishing.com/semana/OKQNZHBHFVEF3ALWFQXNT2GNO4.jpeg)
Eye drops recall FDA warns to immediately stop using 26 types of over, Fda announces recall of eye drops from leading brands over safety concerns. On february 26, 2025, the fda issued a recall notice concerning the sterility of eye ointments produced by brassica pharma pvt.

Your eyedrops could be filled with filthy bacteria. The fda says ‘ultimately industry is responsible for the quality of their products’.
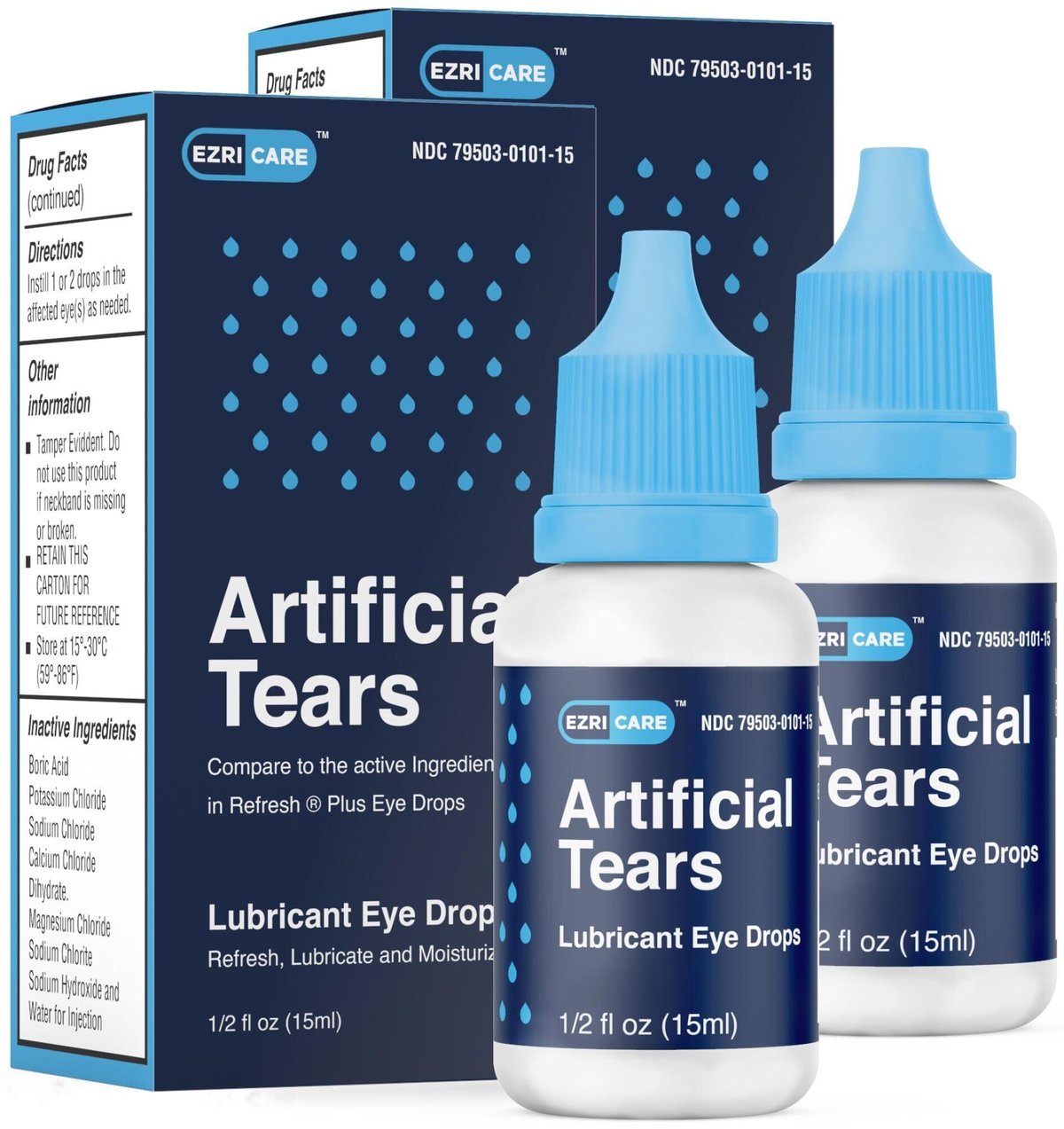
US FDA wants you to ditch these 2 eye drops US FDA wants you to ditch, Announced by the fda on february 26, 2025, the nationwide recall was issued by brassica pharma pvt. In 2023, multiple eye drops were recalled.

More Eye Drops, Ointment Added to Nationwide RecallSee the List, Apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some bottle caps developed cracks, which could compromise. New york cnn — eye ointments sold at cvs and walmart may not actually be sterile, a recall posted by the us food and drug administration warned.
Your eyedrops could be filled with filthy bacteria.
EzriCare Eye Drops Lawsuit — Lawsuit Information Center, Fda is recommending that you discard these products immediately. Over the course of 2023 and 2025, a long list of artificial tear products have been recalled.
Another Death, More Cases of Vision Loss Linked to Tainted Eye Drops, Fda raises concerns over potential infection risk. Announced by the fda on february 26, 2025, the nationwide recall was issued by brassica pharma pvt.

Lágrimas artificiales cuándo y cómo deben utilizarse adecuadamente, New york cnn — eye ointments sold at cvs and walmart may not actually be sterile, a recall posted by the us food and drug administration warned. See below for the eye ointments affected by the recall;
